Source: [Picower News | July 1, 2025]
A first-of-its-kind study in mice reveals that neurons add and shed synapses at a frenzied pace during development to integrate visual signals from the two eyes.
Scientists have long known that the brain’s visual system isn’t fully hardwired from the start—it becomes refined by what babies see—but the authors of a new MIT study still weren’t prepared for the degree of rewiring they observed when they took a first-ever look at the process in mice as it happened in real-time.
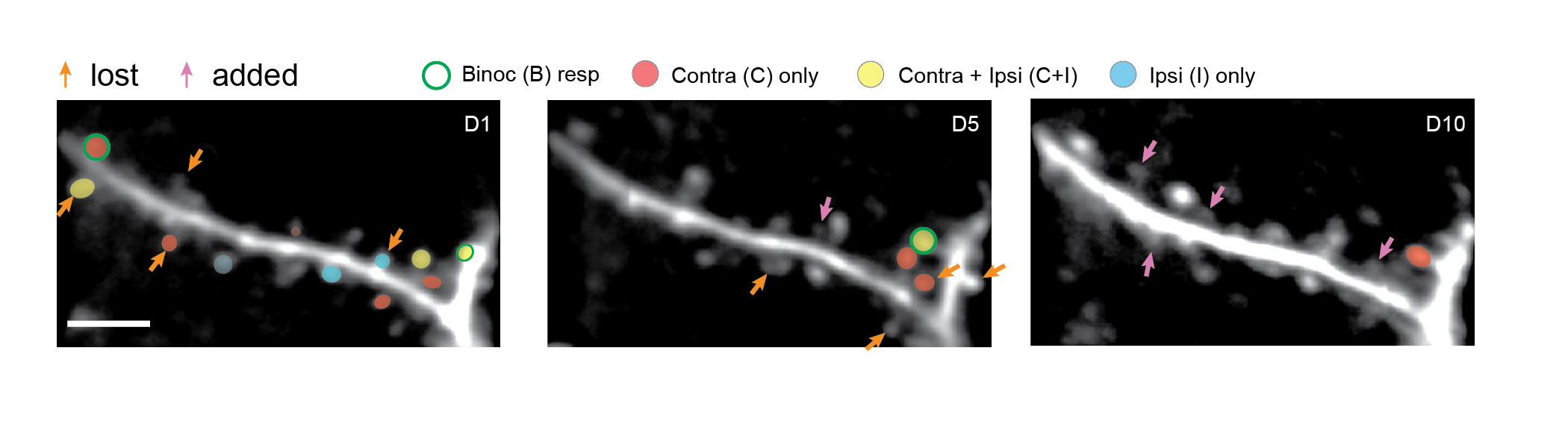
As the researchers in The Picower Institute for Learning and Memory tracked hundreds of “spine” structures housing individual network connections, or “synapses,” on the dendrite branches of neurons in the visual cortex over 10 days, they saw that only 40 percent of the ones that started the process survived. Refining binocular vision (integrating input from both eyes) required numerous additions and removals of spines along the dendrites to establish an eventual set of connections.
Former graduate student Katya Tsimring led the study in Nature Communications, which the team said is the first in which scientists tracked the same connections all the way through the “critical period,” when binocular vision becomes refined.
“What Katya was able to do is to image the same dendrites on the same neurons repeatedly over 10 days in the same live mouse through a critical period of development, to ask, what happens to the synapses or spines on them?,” said senior author Mriganka Sur, Paul and Lilah Newton Professor in The Picower Institute and MIT’s Department of Brain and Cognitive Sciences. “We were surprised by how much change there is.”
Extensive turnover
In the experiments, young mice watched as black and white gratings with lines of specific orientations and directions of movement drifted across their field of view. At the same time, the scientists observed both the structure and activity of the neurons’ main body (or, “soma”) and of the spines along their dendrites. By tracking the structure of 793 dendritic spines on 14 neurons at roughly day 1, day 5 and day 10 of the critical period, they could quantify the addition and loss of the spines and therefore the synaptic connections they housed. And by tracking their activity at the same time, they could quantify the visual information the neurons received at each synaptic connection. For example, a spine might respond to one specific orientation or direction of grating, several orientations, or may not respond at all. Finally, by relating a spine’s structural changes across the critical period to its activity, they sought to uncover the process by which synaptic turnover refined binocular vision.
Structurally, the researchers saw that 32 percent of the spines evident on day 1 were gone by day 5, and that 24 percent of the spines apparent on day 5 had been added since day 1. The period between day 5 and day 10 showed similar turnover: 27 percent were eliminated but 24 percent were added. Overall, only 40 percent of the spines seen on day 1 were still there on day 10.
Meanwhile, only 4 of the 13 neurons they were tracking that responded to visual stimuli still responded on day 10. The scientists don’t know for sure why the other 9 stopped responding, at least to the stimuli they once responded to, but it likely they now served a different function.
What are the rules?
Having beheld this extensive wiring and rewiring, the scientists then asked what entitled some spines to survive over the 10-day critical period.
Previous studies have shown that the first inputs to reach binocular visual cortex neurons are from the “contralateral” eye on the opposite side of the head (so in the left hemisphere, the right eye’s inputs get there first), Sur said. These inputs drive a neuron’s soma to respond to specific visual properties such as the orientation of a line—for instance, a 45-degree diagonal. By the time the critical period starts, inputs from the “ipsilateral” eye on the same side of the head begin joining the race to visual cortex neurons, enabling some to become binocular.
It’s no accident that many visual cortex neurons are tuned to lines of different directions in the field of view, Sur said.
“The world is made up of oriented line segments,” Sur noted. “They may be long line segments; they may be short line segments. But the world is not just amorphous globs with hazy boundaries. Objects in the world—trees, the ground, horizons, blades of grass, tables, chairs—are bounded by little line segments.”
Because the researchers were tracking activity at the spines, they could see how often they were active and what orientation triggered that activity. As the data accumulated, they saw that spines were more likely to endure if a) they were more active, and b) they responded to the same orientation as the one the soma preferred. Notably, spines that responded to both eyes were more active than spines that responded to just one, meaning binocular spines were more likely to survive than non-binocular ones.
“This observation provides compelling evidence for the ‘use it or lose it’ hypothesis,” said Tsimring. “The more active a spine was, the more likely it was to be retained during development.”
The researchers also noticed another trend. Across the 10 days, clusters emerged along the dendrites in which neighboring spines were increasingly likely to be active at the same time. Other studies have shown that by clustering together, spines are able to combine their activity to be greater than they would be in isolation.
By these rules, over the course of the critical period, neurons apparently refined their role in binocular vision by selectively retaining inputs that reinforced their budding orientation preferences both via their volume of activity (a synaptic property called “Hebbian plasticity”) and their correlation with their neighbors (a property called “heterosynaptic plasticity”). To confirm that these rules were enough to produce the outcomes they were seeing under the microscope, they built a computer model of a neuron and indeed the model recapitulated the same trends as what they saw in the mice.
“Both mechanisms are necessary during the critical period to drive the turnover of spines that are misaligned to the soma and to neighboring spine pairs,” the researchers wrote, “which ultimately leads to refinement of [binocular] responses such as orientation matching between the two eyes.”
In addition to Tsimring and Sur, the paper’s other authors are Kyle Jenks, Claudia Cusseddu, Greggory Heller, Jacque Pak Kan Ip, and Julijana Gjorgjieva. Funding sources for the research came from The National Institutes of Health, The Picower Institute for Learning and Memory, and the Freedom Together Foundation.

